How lithium batteries work
In a lithium battery, there is no chemical reaction.
Movement of ions
Lithium battery technology works on the movement of ions.
Lithium ions move in or out of the lithium ferrous phosphate (LiFePO4) compound which coats the positive plate, and in or out of the graphite coating on the negative plate.
If a lithium ion is pushed out of one plate into the electrolyte, another lithium ion must come out of the electrolyte into the other plate.
This process is referred to as intercalation and this is how the lithium ion attaches to the graphite without actually changing its chemical makeup:
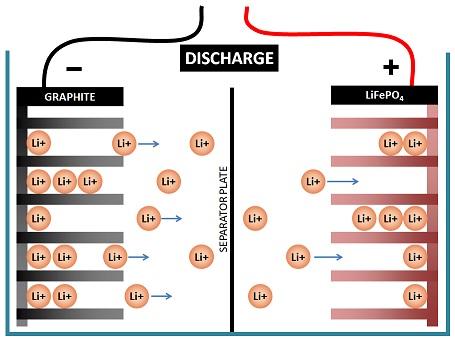
Intercalation
The important thing to note here is that the lithium ion that moves out of one plate is not the same ion that moves into the other plate. The effect is much like the Newton's cradle, a common desk toy:
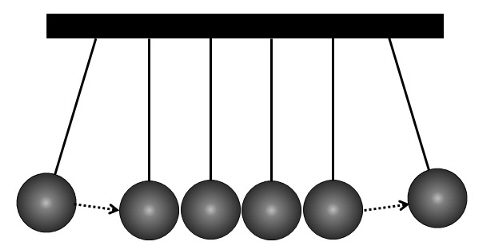
Newton's cradle
The first ball pushes the next ball and its energy is transferred through the balls until it reaches the last ball, which is then pushed away.
Low internal resistance
The simplicity of the process of ion movement, and the low energy consumption of that process, means a lithium battery has a low internal resistance. It's this low internal resistance that results in the fast charging and discharging of a lithium battery. It's also the reason why connecting lithium batteries in parallel results in one working much harder than the others.
Safe lithium voltages
As long as there are some lithium ions left in the lithium compound that coats the positive plate and somewhere for them to link into the graphite coating the negative plate, there are no secondary reactions, as long as the cell voltage stays within the safe range, and this is the key to a long cycle life.
For lithium ferrous cells (liFePO4 and LiYFePO4), this safe range is:
2.8V low voltage
3.6V high voltage
This safe range applies to each and every cell, not an average across all the cells connected in series. This is why the monitoring of cell voltage is so important.
Lithium battery capacity
A quality lithium battery will give you 100% of its advertised capacity without damage or loss of cycle life.
If you want to pull 100 amps from your 100Ah battery, you can have that for 1 hour. You cannot do this with a lead acid battery, which will give you about 20 mins until the battery is flat, not until it’s at 50%.
If you want 50 amps from your 100Ah lithium battery, you can have that for 2 hours.
The standard discharge rate for quality lithium batteries is called 0.5CA. CA means the capacity referred to in amps.
- 0.5CA means 50 amps from a 100Ah battery.
- 1CA means 100 amps from a 100Ah battery.
A quality lithium battery can deliver up to 5CA – this means 500amps from your 100Ah. You won’t get it for very long, but it will give you almost all of the 100Ah stored and will do it without damaging the battery, as long as that minimum 2.8V in any cell is observed.

In order to know why lithium batteries work so well, it helps to understand how lead acid batteries work. The stark difference explains why lithium leads the way in charge and discharge speed, cycle life and longevity.
Many different chemistries come under the banner of 'lithium', but they all have different efficiencies and stability. It's important to know that not all lithium chemistries are suited to RV use.